

Only the superior quality gelatin and HPMC are used to make our capsules, in compliance with U.S. Pharmacopoeia standards. All raw materials are carefully inspected prior to usage. In addition, we only use gelatin from countries that are free from bovine spongiform encephalopathy (BSE). BSE-free certificate is available upon request.

Adding gelatin or HPMC raw materials with purified water into dissolving tank until it’s completely dissolved.

The colorants used are in compliance with EU directives and upon request with the FDA requirements.

We equips with 46 production lines (including 10 fully automatic production lines from Canada). Relying on matured production technique and strict process control to guarantee the quality. Each process contains professionals to inspect in order to ensure best quality.

The capsules are sorted by special inspecting machines which could take pictures for each grain of capsules and automatically filter out defective goods.

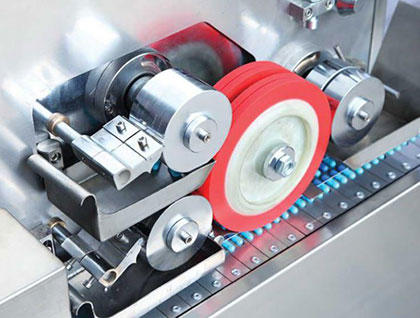
It is equipped with circumferential and axial capsule printing. Thus, we offer the monochrome or double-color printing ink loop and axial capsule printing service of various words and patterns according to the requirements of customers.

QCs manually select the capsules after machine inspection or printing. Then QAs take samples from each batch to pilot laboratory to test the concentration of ethylene oxide, chloroethanol and microorganisms. After the QA inspection is qualified, the quality management department will pass the batch record and issue a certificate of qualification.
The inner packaging material of the direct contact capsules should strictly comply with the relevant requirements of the packaging of the medicinal solid preparation. The outer packaging should have a certain strength enough to protect it from being crushed and deformed during transportation and storage, thus affecting the use of the product.

This product must be sealed and stored in a clean, dry, ventilated warehouse. It should not be stacked in the open air. The best storage conditions are relative humidity of 35%- 65% and temperature of 10°C-25°C.


Tianlong is a professional vegan capsules manufacturer founded since 1987. It is located in Xinchang county of Zhejiang province, which is about 100 km far from Ningbo Port and 300 km from Shanghai port.
